Abstract
Background: Investigational Bruton tyrosine kinase (BTK) inhibitor zanubrutinib has demonstrated greater selectivity vs other TEC and EGFR family kinases in biochemical assays and favorable pharmacokinetic/pharmacodynamic properties in preclinical studies. In phase 1 testing, high plasma concentrations were achieved, resulting in complete and sustained 24-hour BTK inhibition in blood and lymph nodes in patients treated at 160 mg twice daily (bid; Tam et al. Blood 2016;128:642). A recent update of clinical data suggested that complete and sustained 24-hour BTK occupancy is associated with durable responses in patients with non-Hodgkin lymphomas (Tam et al. Blood 2017;130:152). Here, we present updated safety and efficacy data from patients with mantle cell lymphoma (MCL).
Methods: Study AU-003 is a global, open-label, multicenter, phase 1b trial investigating zanubrutinib in patients with B-cell malignancies. After dose escalation, the expansion phase enrolled disease specific cohorts at the recommended phase 2 dose of zanubrutinib (320 mg/day once daily or 160 mg bid). Treatment emergent adverse events (TEAEs) were summarized according to NCI CTCAE v4.03 and response was assessed per International Conference on Malignant Lymphoma criteria (Cheson et al. J Clin Oncol 2014;32:3059). Positron-emission tomographic (PET) scan was not required for response assessment.
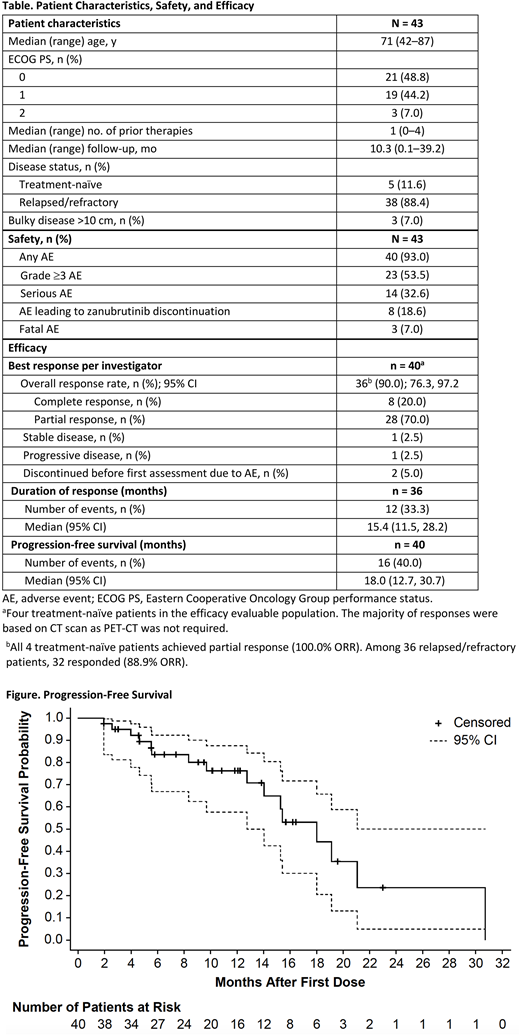
Results: As of 28 Feb 2018, 43 patients were enrolled: 38 relapsed/refractory and 5 treatment-naïve (Table). Median follow-up was 10.3 months (range, 0.1-39.2). Twenty patients have discontinued treatment (12 due to progressive disease; 8 due to TEAEs). Most common TEAEs of any cause (≥15% of patients) included diarrhea (30.2%), petechiae/purpura/contusion (30.2%), upper respiratory tract infection (27.9%), constipation (18.6%), fatigue (18.6%), and rash (16.3%). Two cases of atrial fibrillation/flutter (4.7%) and 3 cases of major hemorrhage (7.0%; renal hematoma, gastrointestinal hemorrhage, and tumor hemorrhage, all grade 3) were reported. Most common grade ≥3 TEAEs of any cause (≥2 patients) included anemia (7.0%), cellulitis (7.0%), pneumonia (7.0%), myalgia (7.0%), neutropenia (4.7%), back pain (4.7%), peripheral edema (4.7%), hypertension (4.7%), and acute kidney injury (4.7%). Fourteen patients experienced ≥1 serious TEAE (SAE) of any cause; SAEs occurring in >1 patient included pneumonia (7.0%) and cellulitis (4.7%). Nine TEAEs led to discontinuation of zanubrutinib in 8 patients: pneumonia, cognitive disorder, antineutrophil cytoplasmic antibody-positive vasculitis and acute kidney injury (same patient), joint effusion, and myelodysplastic syndrome; 3 of the 9 were fatal TEAEs designated by the investigator as unrelated to zanubrutinib including pneumonia, congestive cardiac failure, and cerebral infarction. Three patients who had not yet reached their first response assessment were not evaluable for response. As shown in the Table, overall response rate was 90.0% (n=36/40) including 20.0% (n=8) with complete response. Response was based on computed tomography (CT) scan alone for the majority of patients as PET was not required. The 4 non-responders included 1 with progressive disease, 1 with stable disease, and 2 patients who discontinued because of TEAEs (pneumonia, congestive cardiac failure) before the first response assessment. Median progression-free survival was 18.0 months (Table, Figure).
Conclusions: Zanubrutinib monotherapy was demonstrated to be highly active in patients with relapsed/refractory MCL, with a safety profile consistent with that of previous reports of zanubrutinib.
Tam:Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding. Wang:MoreHealth: Consultancy; Novartis: Research Funding; Dava Oncology: Honoraria; Kite Pharma: Research Funding; Juno: Research Funding; Pharmacyclics: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Research Funding; Acerta Pharma: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Simpson:Celgene: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Bristol-Myers Squibb: Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Sanofi: Research Funding; BeiGene: Research Funding; Merck: Honoraria, Research Funding; Acerta: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Roche: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Amgen: Research Funding, TRAVEL, ACCOMMODATIONS, EXPENSES; Janssen: Honoraria, Research Funding; Novartis: Other: TRAVEL, ACCOMMODATIONS, EXPENSES; MSD: Honoraria. Opat:Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses; Novartis: Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Clinical Trial Support, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Clinical Trial Support, Research Funding; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Clinical Trial Support, Research Funding; Amgen: Research Funding; Mundipharma: Honoraria; Beigene: Other: Clinical Trial Support; Epizyme: Other: Clinical Trial Support; Merck & Co., Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Clinical trial support and travel expenses; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Clinical Trial Support, Research Funding. Cull:Takeda Australia: Other: Travel Expenses; Amgen Australia: Other: Travel Expenses; AbbVie (Australia): Membership on an entity's Board of Directors or advisory committees. Munoz:Alexion: Consultancy; Kite: Consultancy, Honoraria, Speakers Bureau; Gilead: Speakers Bureau; Juno: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy; Janssen: Consultancy; Pfizer: Consultancy; Pharmacyclics: Consultancy, Honoraria; Bayer: Consultancy, Speakers Bureau; Genentech: Consultancy, Honoraria. Phillips:Gilead: Consultancy; Bayer: Consultancy; Seattle Genetics: Consultancy; Pharmacyclics: Consultancy, Research Funding; Abbvie: Research Funding; Genentech: Consultancy. Kim:Novartis: Research Funding; Roche: Research Funding; Celltrion: Research Funding; Takeda: Research Funding; Kyowa-Kirin: Research Funding; Mundipharma: Research Funding; J&J: Research Funding. Hilger:BeiGene (Beijing) Co., Ltd.: Employment, Equity Ownership. Huang:BeiGene (Beijing) Co., Ltd.: Employment, Equity Ownership. Novotny:BeiGene (Beijing) Co., Ltd.: Employment, Equity Ownership. Trotman:PCYC: Research Funding; Janssen: Other: Unremunerated member of Ad Board, Research Funding; Celgene: Other: Unremunerated member of Ad Board, Research Funding; Takeda: Other: Unremunerated member of Ad Board; F. Hoffman-La Roche: Other: Travel to meeting, Unremunerated member of Ad Board, Research Funding; Beigene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.